Staging of Preclinical Myxomatous Mitral Valve Disease in Primary Practice

By Julie Hamilton-Elliott, RCVS and EBVS ® Specialist in Veterinary Cardiology
Myxomatous mitral valve disease (MMVD) is the most common acquired cardiac disease in canine patients, representing over 75% of cardiovascular disease in this species.
Primary care veterinary surgeons commonly encounter patients with MMVD on a daily basis. The detection of a characteristic left apical systolic heart murmur in an older, but otherwise clinically well, small breed dog will understandably raise questions from clients as to the significance of the murmur, whether further investigations should be performed and treatment options available. The gold standard approach to diagnosis, staging and treatment decision making of MMVD is echocardiography. Whilst it is completely reasonable to offer onward referral to an advanced practitioner or specialist in the cardiology, this is not an option for all patients, whether logistically or financially, and with some basic echocardiographic competence, it is possible to diagnose and stage preclinical MMVD in the primary care setting. The purpose of this article is to summarise the standard approach to diagnosis and staging of MMVD in primary practice based on current published guidelines.
Some Pathophysiological Background
MMVD is characterised by histopathological degenerative changes to the mitral valve (MV) apparatus, including the valve leaflets, chordae tendinae and papillary muscles.
Grossly, early valvular lesions consist of small nodules which progress to valvular thickening with some areas bulging or prolapsing towards the left atrium. The chordae tendinae which provide vital support to the MV apparatus, often develop similar degenerative changes and can eventually rupture. Other valves within the heart, particularly the tricuspid, may also be affected by myxomatous change however usually less severely.
The sequelae to the valvular changes is progressive deformation of valve structure eventually leading to reduced valvular coaptation (ability of the valves to close together), resulting in mitral regurgitation or MR (leakage of blood into the left atrium). As the valve degeneration worsens, so too does the volume of MR. Secondary cardiac remodelling develops in response to altered loading conditions and neurohormonal stimulation including eccentric hypertrophy of the atrium and ventricle. Left ventricular dilation can result in annular stretch, leading to secondary MR. Finally, congestive heart failure (CHF) and ventricular myocardial failure will develop in severe cases.
The Typical MMVD Patient
MMVD is a disease of older, small breed (<20Kg) dogs, the prevalence increasing with age and the majority of small breeds showing some evidence of valvular degeneration by 13 years of age. The disease is also 1.5 times more common in males than females. There are notable breed predispositions, particularly the Cavalier King Charles Spaniel (CKCS), with almost all CKCS developing some degree of MMVD within their lifetime. Medium to large breed dogs may also be affected but they tend to demonstrate a slightly different disease progression and the focus of this article is on small breeds.
On physical examination, MR will present as a left sided, apical, systolic heart murmur; when the ventricle pumps (systole), a proportion of blood which should move ‘forwards’ into the aorta, regurgitates ‘backwards’ into the left atrium, resulting in the murmur. Murmur grade is usually proportionate to MR volume/severity until the very late stages of the disease when myocardial failure could reduce murmur intensity. Remember that there are other causes for mitral murmurs (e.g. congenital mitral dysplasia, dilated cardiomyopathy with secondary annular stretch, endocarditis) and you should think about the murmur in the context of the signalment, clinical history and other physical features of the case (e.g. a young dog is unlikely to have degenerative valvular disease).
MMVD Staging
Echocardiography is the gold standard method for diagnosis of MMVD. Complementary diagnostic tests include thoracic radiography and cardiac biomarkers (e.g. proBNP) which are beyond the scope of this article.
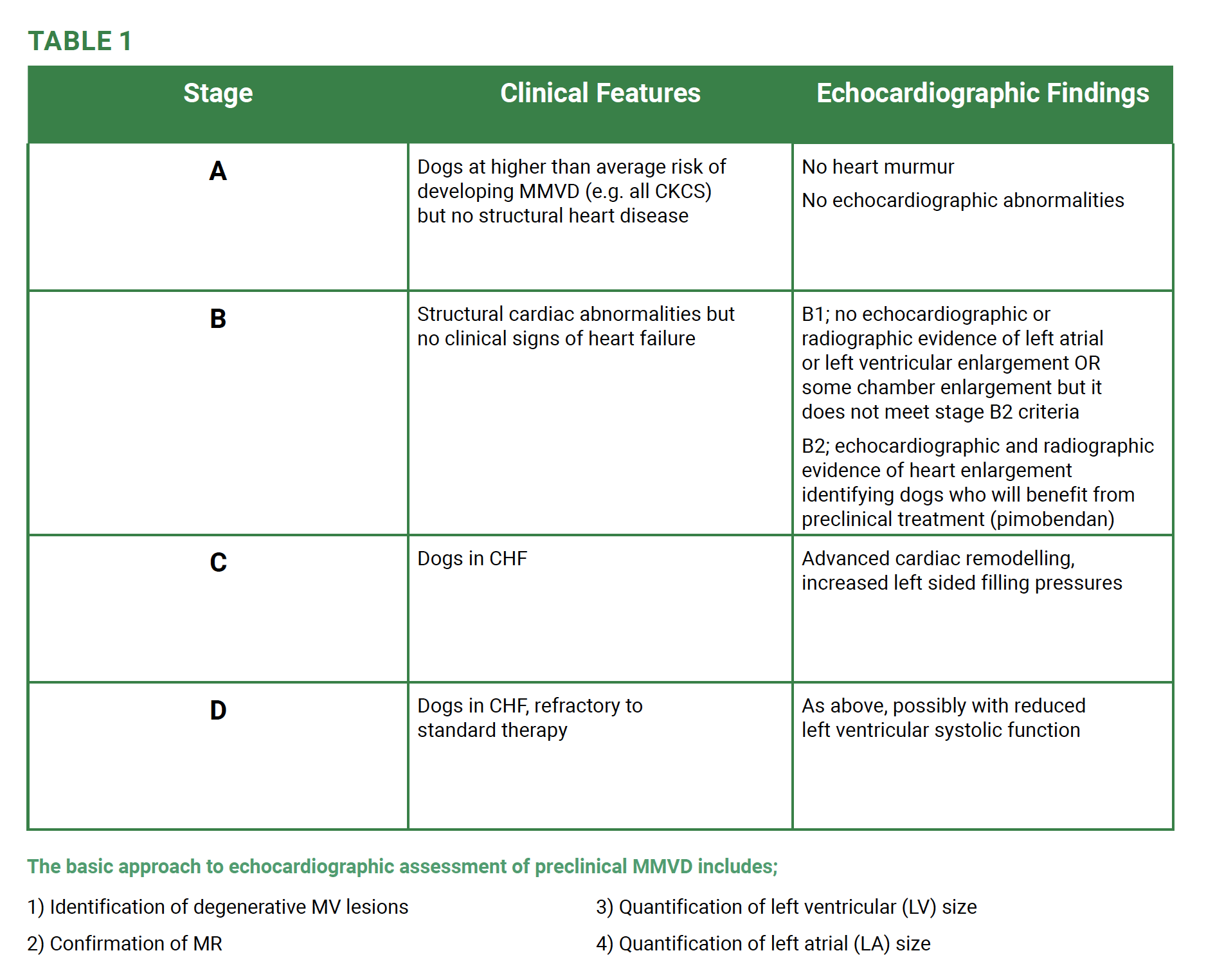
It is important to understand that echocardiography is aimed at confirming a presumptive diagnosis of MMVD but also for disease staging as, contrary to popular opinion, not every dog with preclinical MMVD needs to receive treatment. Current consensus guidelines (Keene et al 2019) for staging of MMVD is summarised in table 1.
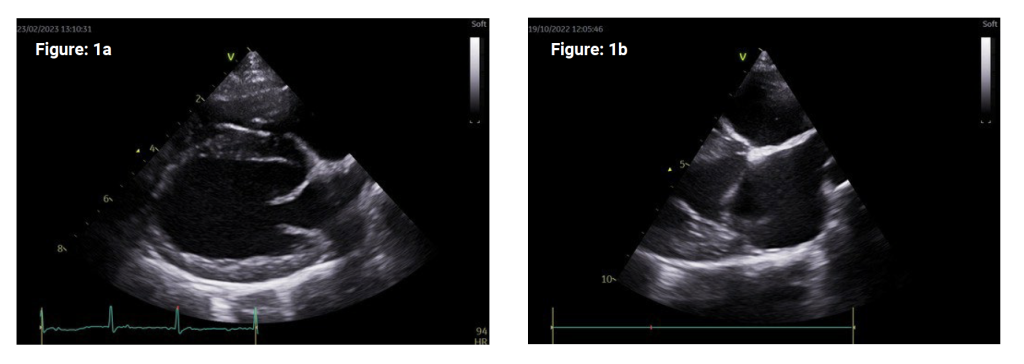
1) Identification of degenerative MV lesions
MV abnormalities can typically be observed on the right parasternal 4-chamber (RP4ch), long axis view (Figure 1a). The sector width can be reduced to include only the MV apparatus and LA in order to increase frame rate in assessing MV motion (Figure 1b).
2D echocardiographic characteristics of MMVD include nodular thickening, particularly associated with the free edges of the valvular leaflets along with thickening of the chordae tendinae. As the disease progresses, valvular prolapse or thickening of one or both MV leaflets may be observed. Mitral valve prolapse (MVP) is characterised by abnormal displacement or buckling of the MV leaflets into the LA during systole. Methods exist to estimate the degree of MVP but this is not essential to staging of MMVD.
2) Confirmation of MR
Ideally colour flow Doppler should also be applied to confirm and aid in quantification of the severity of MR. However, the presence of a left apical systolic murmur on physical examination combined with echocardiographic degenerative MV changes means this is not an essential step and colour Doppler may not be available on every ultrasound machine.
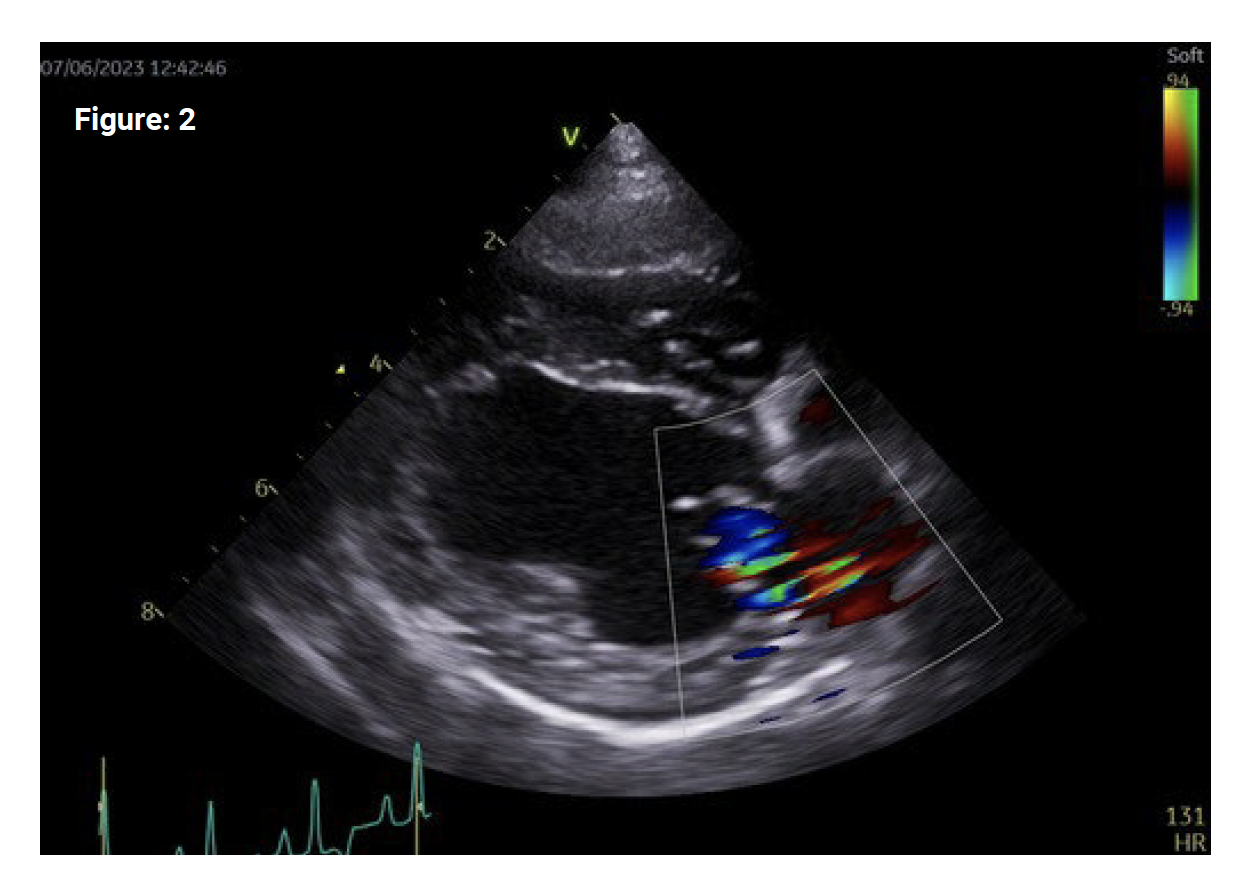
On the same RP4ch long axis image focused on the LA chamber, colour flow Doppler can be applied with the colour box expanded to include the MV annulus and entire LA (figure 2). MR will be demonstrated as an aliased/turbulent jet of colour which is usually eccentric, coursing towards the interatrial septum or LA free wall with one or multiple jets present. Make sure to adjust the baseline on the colour bar (increase the Nyquist limit) to an appropriate velocity (0.7-1m/s) to minimise excessive aliasing and improve delineation of the regurgitant jet.
The presence of MV lesions and MR are common to both stage B1 and B2 MMVD. The factors which differentiate these two stages are the presence of a certain degree of both LV and LA remodelling. The echocardiographic cut-offs for stage B2 disease are derived from the EPIC study (Boswood et al 2016) which showed that dogs with preclinical MMVD and significant remodelling of the LV and LA had delayed progression to development
of CHF if they received pimobendan
(>15 months longer than placebo). Therefore, the stage B2 criteria for
heart enlargement identifies dogs
that are likely to benefit substantially from treatment before the onset of CHF. Criteria includes;
– Body weight <20kg
– Murmur intensity ≥ 3/6
– Echocardiographic LA:Ao ratio in the right-parasternal short axis (RPSA) view in early diastole ≥ 1.6
– Left ventricular internal diameter in diastole, normalised for body weight (LVIDDn) ≥ 1.7
– Breed-adjusted radiographic vertebral heart score (VHS) >10.5
deally all of these criteria should be met before initiating treatment which means we should perform both an echocardiographic study and thoracic radiography on patients with suspected MMVD. However, thoracic radiography often requires chemical restraint and carries additional cost. The consensus statement advises that of all the criteria echocardiographic evidence of LA and LV enlargement is considered to be the most reliable way to identify dogs who will benefit from therapy. Therefore, whilst it may be useful to achieve a ‘baseline’ thoracic study for newly diagnosed dogs, it is acceptable to perform only echocardiography, provided the measurements attained are accurate.
Note also that a stage B2 patient technically needs a heart murmur ≥ 3/6 in order to meet all the criteria. Whilst it is reasonable to investigate any new cardiac murmur (particularly if the owner wants a definitive diagnosis), there is argument to say a dog with a lower grade heart murmur does not require further disease staging as the volume of MR present is unlikely to be sufficient to have resulted in significant secondary remodelling and the patient is unlikely to require therapy. In this case, serial monitoring of the heart murmur is not unreasonable (for example, repeat auscultation every 3-6 months) with further investigations delayed until a grade 3/6 murmur is identified, particularly if there are financial limitations. Remember, however, this applies only to mitral murmurs and only in dogs with a strong clinical suspicion for MMVD (i.e. older, small breed dogs). Medium to large breed dog with a low grade heart murmur could have significant cardiac disease (e.g. cardiomyopathy). The owner must be informed that MMVD cannot be diagnosed on auscultation alone.
We now know that to echocardiographically differentiate stage B1 from stage B2 dogs we need to quantify LV and LA size;
3) Quantification of LV size
The RP4ch view can provide a subjective sense of LV size and shape. With progressive disease the LV will dilate and become subjectively spherical/rounded, consistent with volume overload. Quantitative measurements of left ventricular size and volume attained from the RP4ch view are also commonly utilised by more experienced echocardiographers but are not included in the current recommendations for disease staging and so are not discussed here.
The echocardiographic measurement required for MMVD staging is the LVIDdn. This is attained from 2D motion (M) mode of the LV from the RPSA view at the level of the papillary muscles. It can be useful to attain the short axis view at the level of the MV leaflets (the ‘fish mouth’ view – Fig 3a) and fan slightly downwards until only the papillary muscles are visible. On the 2D image, make the LV cavity is as symmetrical as possible. The M mode cursor is then positioned through the centre of the papillary muscle and must bisect the structures as symmetrically as possible (figure 3b). Oblique placement of the cursor (an example is provided in figure 3c) will result in unreliable/useless measurements. In this position, the M mode will produce the standard LV study (figure 4).
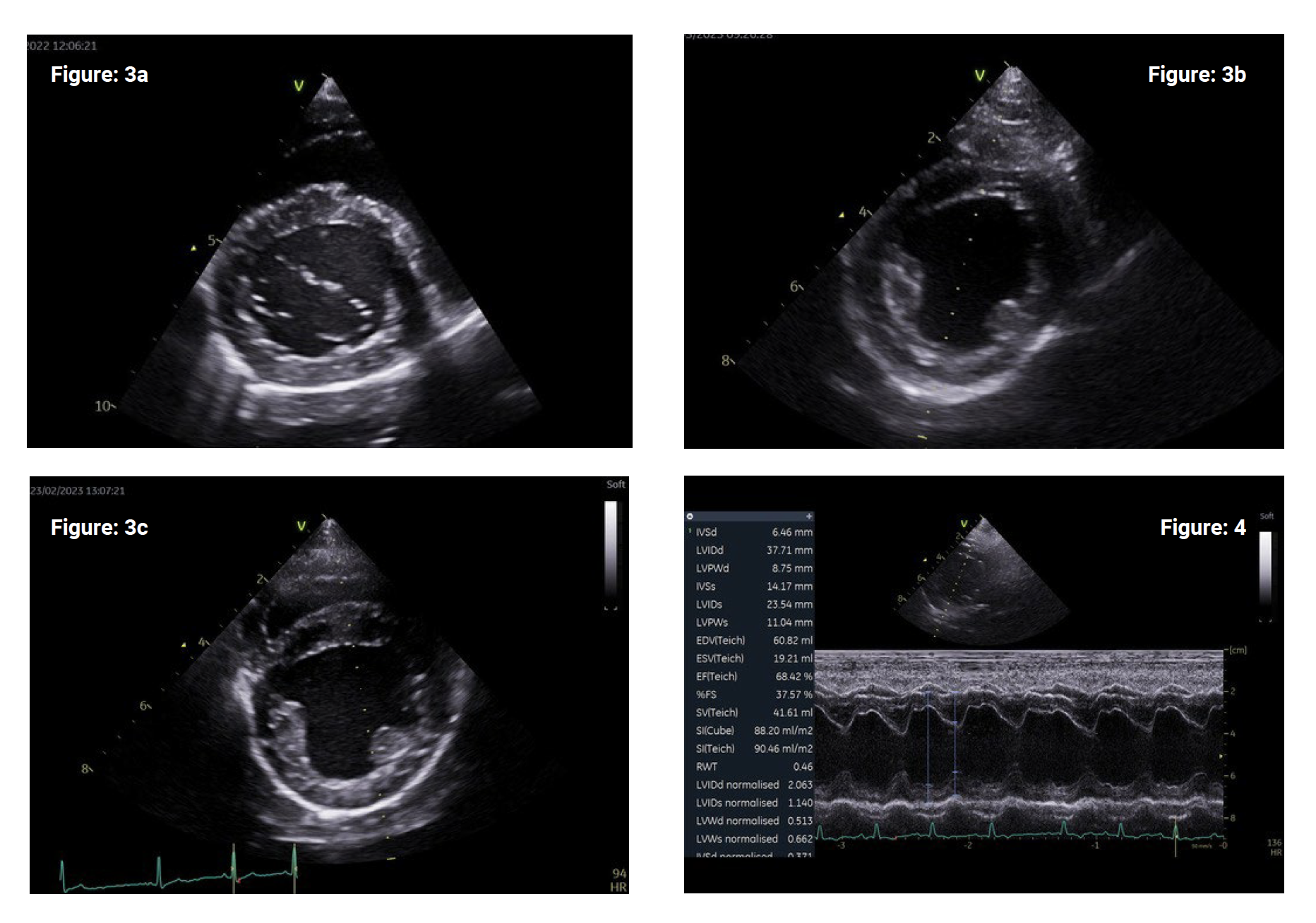
Once the M mode image is produced, use the ‘leading edge to leading edge’ method so the proximal endocardium only is included in each measurement (figure 4 – blue lines). Several measurements of the left ventricular walls and chamber can be taken from the LV study but for MMVD staging we are focusing on the left ventricular internal diameter in diastole (LVIDd) in end diastole. Ideally, simultaneous ECG should be used in order to achieve accurate diastolic dimensions coinciding with the correct phase of the cardiac cycle. End-diastolic is defined as the start of the QRS complex (figure 4 – red dotted line). If you do not have simultaneous ECG you can estimate the maximum dimensions of the LV (when it is at its most full) however of course this will not be as accurate which should be borne in mind for ambiguous cases.
The measured LVIDd measurement can be indexed for body weight (BW) using allometric scaling (Cornell et al. 2004). We can then work out if the LV is dilated for the dog’s BW (i.e. the index normalises for BW). The formula for normalising LVIDD to BW = LVIDd(cm)/weight(kg)0.294. Note that the normal canine LVIDdn_index is actually 1.27-1.85 (2.5-97.5 percentile) however the 95th percentile in the Cornell study was 1.73 hence the EPIC inclusion criteria defined LVIDdn >1.7 as representing left ventricular dilation.
Some ultrasound machines will calculate LVIDdn automatically or you can calculate manually. If you are unable to calculate LVIDdn directly, you can consult the original (Cornell et al 2004) publication which contains a table of mean M-mode measurements from healthy dogs with prediction intervals so you can see if your case exceeds normal reference ranges. The recent consensus guidelines (Keene et al 2019) also includes this table for dogs weighing 1-20kg. Finally, Heart Vets have a handy calculator on their website: https://heartvets.co.uk/mitral-valve-disease-staging-epic-trial/.
4) Quantification of LA size
Similar to 2D assessment of the LV, LA size can be subjectively and quantitively assessed on the RP4ch view. There are certain subjective features to look out for, including the appearance of the interatrial septum – it should be ‘straight’ and not bowed to the right or left implying elevated left or right atrial pressures respectively. The attached pulmonary vein should be roughly similar in dimension to the right branch of the pulmonary artery posterior to the vein. The maximum diameter of the left atrium in the long axis view (LAmax) can be used for serial monitoring of LA size in individual patients. Again, more advanced echocardiographic techniques to assess LA size, volume and function exist which are not included in MMVD staging and so not covered here.
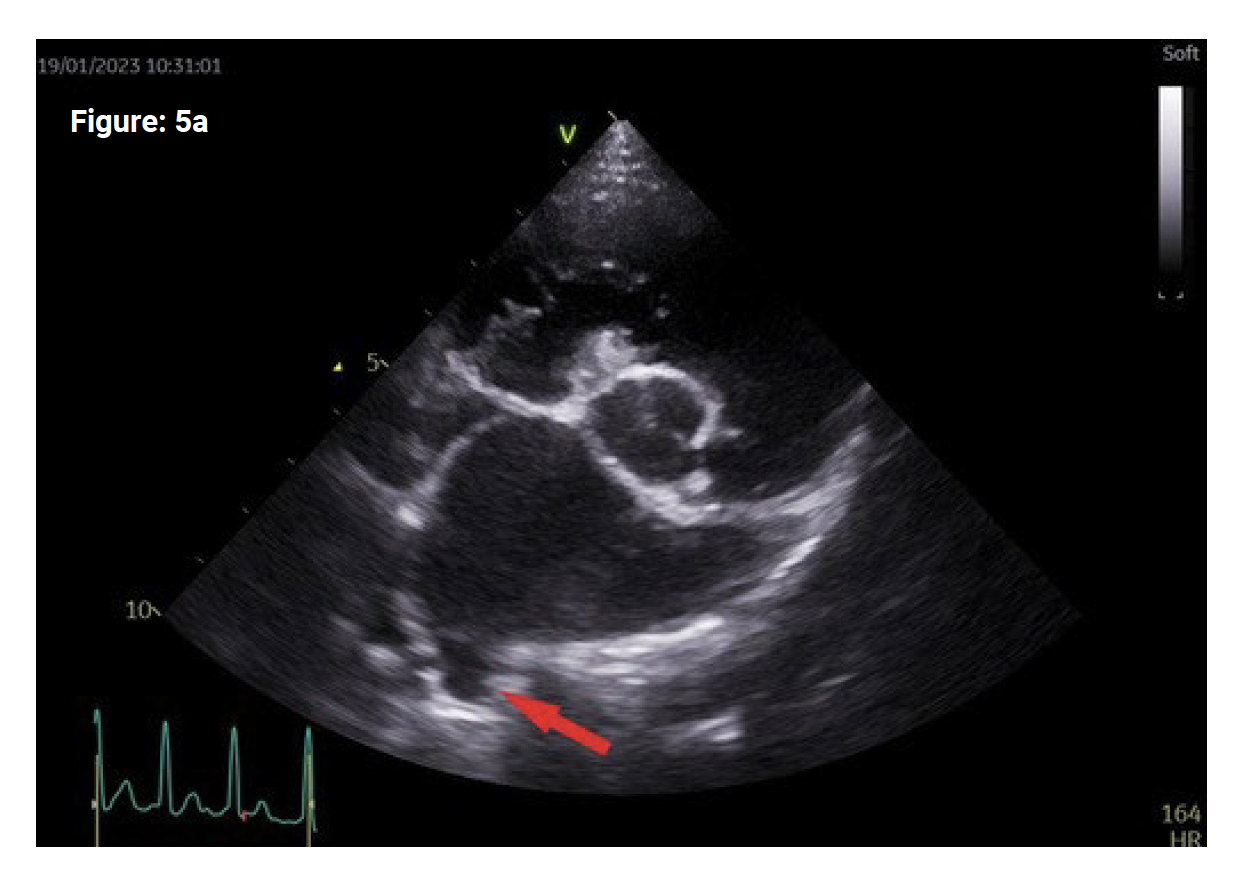
The echocardiographic measurement of LA size for MMVD staging is the LA/Ao ratio. To achieve this, a RPSA view at the level of the aortic valves (the ‘Mercedes Benz’ sign) is required with a well-defined/demarcated LA border. An attached pulmonary vein will often be seen entering the left atrium (figure 5a – red arrow).
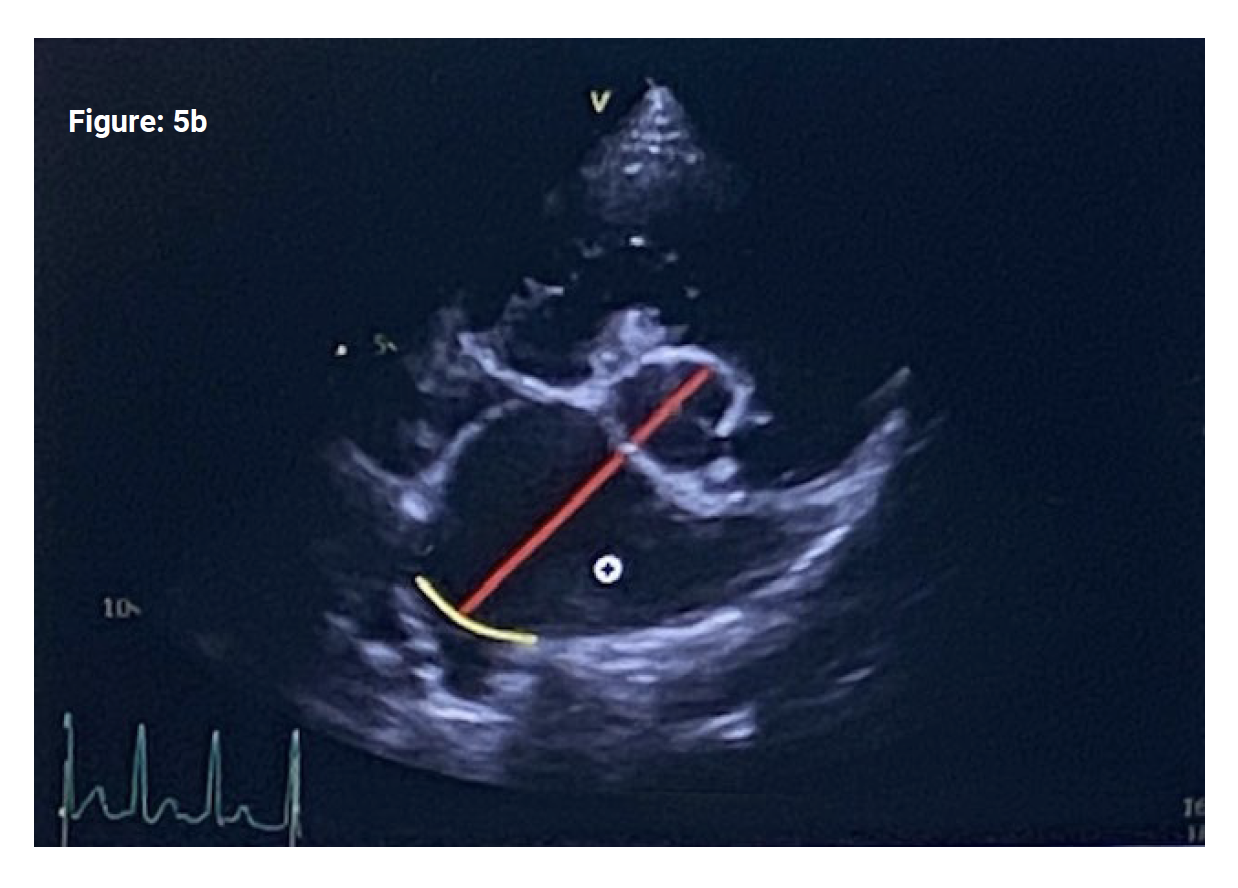
From a still image, the frame immediately following aortic valve closure is selected, ensuring the aortic valve cusps are visible and the LA border is clear. The image is measured at this phase of the cardiac cycle to make sure the LA is being measured in early diastole (when it is at its biggest). This also correlates roughly with the end of the T wave on the simultaneous ECG but the aortic valves must have just closed. The ‘inner edge to inner edge’ method is used to measure aortic diameter (figure 5b) and then LA short axis diameter along the same plane. The diastolic short axis LA/Ao ratio may be automatically calculated on some machines or can be easily calculated manually. The cut off for LA dilation is ≥1.6.
Whilst simple to achieve, the LA/Ao measurement is notoriously open to error and poor repeatability. Problems and pitfalls include;
~ Being too dorsal, so the aortic valves (the ‘Mercedes Benz sign’) are not visible. Try to fan slightly downwards in order to bring all valve cusps into view.
~ Failure to optimise the LA so it is excessively small. Sliding the probe up the chest wall (dorsally) can help to improve LA optimisation.
~ Difficulty identifying the border of the LA and entering pulmonary vein – this often results in overestimation of LA size. Extrapolating the visible LA border through the pulmonary vein (Fig 5b – yellow line) can help but is obviously open to error.
~ Measuring both the aortic and LA diameter at the wrong phase of the cardiac cycle. We should be aiming for consistency as to when measurements are taken both within the same patient and for all patients so our decision making is consistent with ourselves and other clinicians. The consensus guidelines states LA/Ao ratio should be measured in early diastole (see above) so this should be followed by everyone staging dogs with MMVD.
nd other clinicians. The consensus guidelines states LA/Ao ratio should be measured in early diastole (see above) so this should be followed by everyone staging dogs with MMVD.
As a reminder, it is only the dogs with preclinical MMVD who have reached stage B2 criteria who will benefit from pimobendan (dose 0.25-0.3mg/kg BID). To be classified as stage B2, BOTH LA and LV dimensions must meet the specified criteria. If only the left ventricle is dilated, for instance, then this patient is stage B1. Currently there is no evidence that dogs in stage B1 will benefit from pimobendan.
Conclusion
The objective of this article was to summarise staging of preclinical MMVD for the general practitioner based on currently published guidelines. For further information on current recommendations for disease staging (both preclinical and clinical) in addition to treatment guidelines, I recommend the (Keene et al 2019) consensus guidelines (which are open access) as a great resource. This article is certainly not intended to be a summary of all the complexities of staging and prognostication of MMVD however I hope it is helpful and if you have any questions please do not hesitate to contact your local friendly cardiologist!
References
Boswood A, Haggstrom J, Gordon SG et al (2016). Effect of pimobendan in dogs with preclinical myxomatous mitral valve disease and cardiomegaly: the EPIC Study – a randomised clinical trial. J Vet Intern Med. 30(6); 1765-1779
Cornell CC, Kittleson MD, Della Torre P et al. (2004). Allometric scaling of M-mode cardiac measurements in normal adult dogs. J Vet Intern Med. 18(3): 311-321
Keene BW, Atkins CE, Bonagura JD et al. (2019). ACVIM consensus guidelines for the diagnosis and treatment of myxomatous mitral valve disease in dogs. J. Vet Intern Med. 33:1127-1140








